Exploring Phalloidin TRITC for Actin Visualization


Intro
In the realm of cellular biology, the ability to visualize structures at the microscopic level plays a pivotal role in advancing our understanding of cellular dynamics. One of the most prominent tools for this purpose is phalloidin TRITC, a fluorescent dye renowned for its affinity to actin filaments. Actin, a crucial component of the cytoskeleton, is involved in various cellular processes including shape maintenance, motility, and division. This article aims to unravel the intricacies surrounding phalloidin TRITC by dissecting its chemical properties, application techniques, and its implications across different research fields.
The discussion initiates with the mechanics of actin polymerization, highlighting why understanding this process is vital for studying cellular behavior. Following that, we delve into the significance of fluorescent tagging with TRITC, looking at how it enhances visualization under a microscope. The piece also examines advancements in imaging technologies that have revolutionized the way scientists observe cellular structures. However, it's not all sunshine and rainbows—limitations and considerations necessary for effective use of phalloidin TRITC will also be addressed. Finally, readers can expect to gain valuable insights into how this chemical contributes to the expanding field of cellular dynamics and morphology.
As we embark on this exploration, it will become evident that phalloidin TRITC is not just a mere tool but a cornerstone in the pursuit of knowledge within cellular biology.
Methodology
Overview of research methods used
Research in this area often leans heavily on a combination of experimental and observational methodologies. Key strategies include:
- Fluorescence Microscopy: This is the primary technique employed to visualize actin filaments tagged with phalloidin TRITC. Image analysis software complements this by assisting in quantifying the intensity of fluorescence.
- Control Experiments: Including untreated samples to establish baselines against which treated samples can be compared ensures robust conclusions.
Data collection techniques
Data is often gathered using a carefully crafted protocol:
- Sample Preparation: Cells are cultured and treated with phalloidin TRITC. Proper fixation is crucial for preserving cell structure during observation.
- Microscopy: The prepared samples are analyzed under specific fluorescence wavelengths which excite TRITC.
- Data Analysis: The acquired images are then analyzed, often utilizing software to gauge the distribution and density of actin filaments.
This methodology not only enables researchers to observe actin dynamics in real-time but also strengthens the validity of findings through meticulous controls and repeatable processes.
Future Directions
Upcoming trends in research
The field is moving rapidly, with emerging technologies paving the way for new discoveries:
- Enhanced Imaging Techniques: Super-resolution microscopy techniques, like STED and SIM, are gaining ground allowing for even finer resolution of cellular structures.
- Live-Cell Imaging: This technique continues to gain attention, providing insights into dynamic processes as they occur in living cells—a landmark advancement.
Areas requiring further investigation
Despite the progress made, numerous avenues still demand attention:
- Actin Behavior in Disease States: Further understanding how actin dynamics change in pathological conditions could be groundbreaking.
- Interactions with Other Cytoskeletal Elements: Exploring how actin interacts with microtubules or intermediate filaments remains a pursuit that necessitates deeper examination.
"Understanding the fundamentals of actin dynamics is paramount for capturing the essence of cellular life."
In summary, phalloidin TRITC stands as a beacon in the exploration of cellular structures. Through advanced methodologies and ongoing research, its contributions continue to illuminate the complexities of cellular dynamics, propelling the biological sciences forward.
Intro to Phalloidin TRITC
The significance of phalloidin TRITC in cell biology cannot be overstated. This specialized dye plays a pivotal role in the visualization of actin filaments, which are integral to understanding cellular structure and function. Researchers leverage its unique properties to delve deeper into cellular dynamics, making it a cornerstone in the toolkit of cell biologists. The application of phalloidin coupled with TRITC allows scientists to observe the perennial dance of actin polymerization—a process that underpins various cellular processes from motility to division.
Definition of Phalloidin and TRITC
Phalloidin is a toxin derived from the Amanita phalloides mushroom, known for its ability to bind specifically to filamentous actin (F-actin). This binding is incredibly stable, allowing for prolonged observation of actin structures. The TRITC portion refers to a fluorescent tag, which stands for Tetramethylrhodamine Isothiocyanate. When phalloidin is conjugated with TRITC, it emits bright red fluorescence under specific light wavelengths. This makes it an effective tool for live and fixed cell imaging, helping researchers to visualize the intricate actin framework within cells.
The Origin of Phalloidin
Phalloidin was first isolated in the 1930s from the Amanita phalloides mushroom, often called the death cap due to its toxicity. Its discovery opened avenues in cellular research, as the compound's preference for actin quickly established it as a key player in understanding cytoskeletal dynamics. The ability to label actin filaments reliably has allowed scientists to elucidate various cellular mechanisms that hinge upon these structures. Over the decades, it has become more refined, with modern conjugation techniques enhancing its applications in microscopy and other imaging methodologies.
Understanding TRITC Tagging
TRITC tagging involves attaching a fluorescent group that has distinct light absorption and emission properties. The brilliance of TRITC lies in its ability to be excited by green light and subsequently emit a vivid red hue. This process allows for the precise imaging of actin filaments within complex cellular environments. When cells are treated with phalloidin TRITC, scientists can track changes in actin dynamics—changes that might occur due to various stimuli or during specific biological processes. The choice of this particular fluorescent dye also holds significance, as its stability minimizes the risks of photobleaching during imaging sessions, leading to clearer results and reproducibility in experiments.
The key advantage of using phalloidin TRITC is its ability to offer an unparalleled view of the cell's cytoskeletal architecture, a view that is indispensable for deciphering the roles of actin in various biological processes.
Understanding the fundamentals of phalloidin and TRITC is essential for researchers looking to leverage these tools in their investigations. It not only enhances experiment accuracy but also propels forward the understanding of cellular mechanisms.
Mechanism of Action
Understanding the mechanism of action for phalloidin TRITC is essential for grasping its role in cell biology. This section delves into how phalloidin interacts with actin, the integral part of the cellular structure, as well as the dynamics surrounding actin polymerization. A robust comprehension of these aspects not only clarifies the utilization of phalloidin TRITC but also enhances the evaluation of its applications in varied biological contexts.
How Phalloidin Binds to Actin
Phalloidin is a remarkable compound that binds specifically to filamentous actin (F-actin). The binding mechanism hinges on the affinity of phalloidin for the actin polymer structure. It promotes stabilization of the filaments, which is crucial for preserving the cellular cytoskeleton. The binding occurs in a unique manner that favors the assembled state of actin while preventing depolymerization.
In practical terms, when cells are treated with phalloidin, it results in brightly fluorescing filaments that can be visualized under a fluorescence microscope. This specificity is what makes phalloidin an invaluable tool in studying various cellular processes, from signaling pathways to mechanical properties.
"The targeted binding of phalloidin to actin provides researchers a clear view of intracellular architecture, essential for understanding myriad cellular functions."
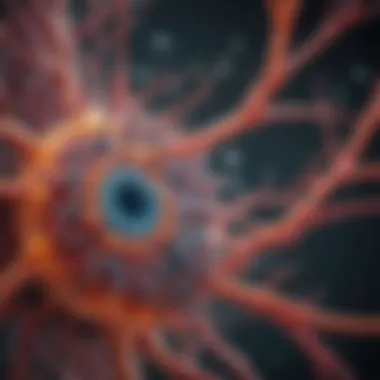

Role of Actin in Cellular Structure
Actin plays a pivotal role in maintaining cellular structure, and its importance cannot be understated. As a major component of the cytoskeleton, actin filaments give cells their shape and aid in movement. They form a complex network that supports the plasma membrane, enabling cell integrity under physical stress. Moreover, actin interactions with myosin drive muscle contractions and cellular motility.
In addition to structural roles, actin is involved in critical processes like cell division and intracellular transport. Understanding how phalloidin affects actin dynamics allows scientists to draw conclusions about cellular behavior and morphology in different states of health and disease.
Polymerization Dynamics of Actin
Actin polymerization is a dynamic and highly regulated process that influences cellular morphology and function. The polymerization occurs in a series of steps: nucleation, elongation, and steady state. Phalloidin’s influence on this process is profound, as it not only stabilizes the filaments but also shifts the equilibrium between actin monomers and filaments.
In essence, when phalloidin is introduced into cells, it triggers an accumulation of actin filaments, disrupting the balance that normally leads to continuous turnover of actin structures.
This enhances our understanding of how alterations in actin polymerization dynamics correlate with various cellular behaviors such as migration, proliferation, or differentiation.
Additionally, investigating phalloidin's impact on polymerization can lead to insights regarding diseases characterized by cytoskeletal abnormalities.
In summary, unraveling the mechanisms of how phalloidin binds to actin, the crux of cellular structure, and the intricacies of polymerization dynamics provides a clear view into the underlying processes that govern cell function. This knowledge is paramount for researchers striving to unlock new pathways in cellular biology.
Applications in Scientific Research
The exploration of phalloidin TRITC in scientific research is not just a matter of academic curiosity; it’s a pathway to understanding the cellular world in detail. By focusing on how actin filaments function and interact within cells, researchers can glean insights into a myriad of cellular processes. This section discusses the pivotal aspects of phalloidin TRITC applications, including visualization techniques, insights into cell motility, and the broader implications of imaging cellular processes.
Visualization Techniques in Cell Biology
Visualization of cellular structures is paramount in cell biology, and the use of phalloidin TRITC stands out in this domain. It allows for highly specific labeling of actin filaments, illuminating these key structures against a background of other cellular components. There are several visualization techniques employed in conjunction with phalloidin TRITC, including:
- Fluorescence Microscopy: This commonly used method is vital for observing the presence and dynamics of actin filaments in live and fixed cells. The bright red fluorescence emitted from TRITC enables clear differentiation of actin structures, even in complex cellular environments.
- Confocal Microscopy: By employing optical sectioning, confocal microscopy provides clearer and sharper images of actin filaments. This technique minimizes background interference, allowing researchers to visualize actin localization accurately.
- Super-Resolution Microscopy: Although more advanced and technically demanding, this method offers unparalleled detail in visualizing actin filament organization. Combining phalloidin TRITC with super-resolution techniques empowers researchers to discern fine structural features that might otherwise go unnoticed.
Such techniques help in unraveling the intricate organization of actin filaments within various cell types, aiding in the understanding of their functional roles.
Use in Understanding Cell Motility
Phalloidin TRITC also plays an essential role in studying cell motility, a critical aspect of many biological processes such as wound healing, immune responses, and cancer metastasis. Actin dynamics are integral to motility, and staining with phalloidin TRITC provides a window into these processes.
Consider examples like:
- Lamellipodia and Filopodia Formation: Phalloidin TRITC can highlight these protrusive structures, which are crucial for cell movement. Researchers often observe changes in the actin cytoskeleton's arrangement during cell migration using this staining method.
- Migration Assays: When combined with imaging techniques, cell motility can be quantitatively assessed, allowing researchers to measure displacement and speed under various experimental conditions.
Understanding how actin filaments contribute to cell motility elucidates not only fundamental cell biology but also has implications for therapies targeting metastasis.
Exploring Cellular Processes through Imaging
The intrinsic nature of phalloidin TRITC to bind to actin allows for broader exploration into various cellular processes via imaging. This opens avenues for studying:
- Cell Division: Actin dynamics during cytokinesis are significant. Phalloidin TRITC staining aids in visualizing the contractile ring, a structure critical for successful cell division.
- Intracellular Transport: Actin filaments serve as tracks for transporting organelles. By visualizing actin with phalloidin TRITC, one can gain insights into the mechanisms underlying the movement of vesicles and other cargo.
- Signal Transduction Pathways: Actin plays a role in cellular signaling. Observing how actin distribution changes during signaling events informs researchers about the interaction between cytoskeleton dynamics and cellular responses.
In summary, phalloidin TRITC is not just a labeling tool; it serves as a lens through which researchers can examine the complex and dynamic world of cellular function.
Insights into cellular mechanisms are pivotal for advances in medicine, biology, and beyond. Understanding the role of actin through visualization techniques can lead to breakthroughs in treating diseases that stem from cellular dysfunction.
Advanced Imaging Techniques
Advanced imaging techniques are crucial in studying cellular dynamics, especially when using Phalloidin TRITC for actin staining. These methodologies provide intricate details that allow researchers to observe complex cellular structures and behaviors. The precise visualization of actin filaments is essential, as it underpins many biological processes such as cell division, motility, and morphology. Thus, an in-depth understanding of these imaging techniques enables researchers to glean pivotal insights into cellular functioning and its underlying mechanisms.
Confocal Microscopy Applications
Confocal microscopy is a standout in the realm of imaging technologies. It significantly enhances the resolution of the images compared to traditional fluorescence microscopy. By utilizing a laser to illuminate samples while capturing images at various depths, it aids in obtaining high-resolution, three-dimensional representations of cells. This depth of information is particularly beneficial when analyzing the organization of actin filaments within cells.
The following points highlight the advantages of confocal microscopy in the context of using Phalloidin TRITC:
- Reduced Background Noise: The laser-based system effectively decreases extraneous light, resulting in clearer visualization of actin filaments.
- 3D Reconstruction: By compiling images taken at different depths, researchers can render a three-dimensional structure, offering a comprehensive view of how actin fibers are arranged.
- Live Cell Imaging: This method allows scientists to observe cellular processes in real-time, revealing the dynamic behaviors of actin during critical moments like cell migration or division.
Fluorescence Microscopy Insights
Fluorescence microscopy plays an indispensable role in studying actin structures tagged with TRITC. This technique capitalizes on the specific emission of light from fluorescent molecules when exposed to a specific wavelength. It plays well with phalloidin, which is a smart choice since it selectively binds to F-actin.
Researchers have noted several key insights through fluorescence microscopy:
- Specificity: The selectivity of phalloidin for filamentous actin allows researchers to distinctly visualize actin networks without interference from other cellular components.
- Quantitative Analysis: Advanced fluorescence microscopy techniques facilitate quantitative analysis of actin distribution, amount, and localization, which is vital for drawing conclusions about cellular processes.
- Multiplexing Capability: Researchers can combine multiple fluorescent markers, allowing concurrent observation of actin and other structural or functional proteins. This multiplexing capability enables a more holistic understanding of cellular mechanics.
High-Resolution Imaging with TRITC
The advent of high-resolution imaging technologies, particularly those compatible with TRITC, has ushered in a new era for photonic studies in cell biology. TRITC emits a vivid red fluorescence that stands out against less intense backgrounds, making it exceptionally useful for discerning intricate actin patterns.


Here are several factors that underline the importance of high-resolution imaging:
- Detailing Actin Architecture: High-resolution techniques allow scientists to discern even minute anomalies in actin organization, which could indicate underlying pathological conditions.
- Temporal Resolution: Fast imaging capabilities enable researchers to track actin dynamics over time, uncovering how these structures respond to changes in the cellular environment.
- Potential for Super-Resolution Techniques: With advancements such as STED (Stimulated Emission Depletion) microscopy, researchers can surpass the diffraction limit of conventional optics, allowing visualization at the nanometer level.
"Advanced imaging techniques reveal the hidden world within cells, transforming our understanding of actin's role in diverse biological functions."
Considerations for Effective Use
When working with phalloidin TRITC, understanding the nuances of its effective use is critical. This section dives into essential considerations that can greatly influence the quality of actin staining and ultimately the interpretation of cellular dynamics.
Concentration and Incubation Times
Getting the right concentration of phalloidin TRITC is akin to hitting the bullseye in archery; too low, and you might miss the mark, while too high could obscure the target altogether. Generally, concentrations between 0.1 to 1.0 µM are suggested for optimum performance. It's crucial to empirically determine the best concentration for specific cell types as they often react differently.
Incubation times also play a significant role. A typical protocol may include a 30-minute to 1-hour incubation at room temperature, but some experimental conditions might demand longer times. Vegetative cells could need less time, while differentiated cells often require a bit more. Just like a simmering pot, leaving it too long can lead to undesired effects such as increased background noise or nonspecific staining.
Choice of Fixatives and Conditions
The choice of fixatives can be the make-or-break factor in your experiment. Formaldehyde is a common choice, but alternatives like paraformaldehyde can offer a better preservation of fine cellular structures. The cells must be adequately fixed without excessive cross-linking; otherwise, the actin filaments might not be accessible for effective binding of phalloidin. Optimal conditions, such as pH and temperature, can also not be brushed aside. A neutral pH around 7.4 is often recommended, as it fits right into the physiological range for cells, ensuring that they are as close to their natural state as possible.
"The selection of appropriate fixatives is not just a technical detail – it can be the difference between clear results and a jumbled mess."
Minimizing Photobleaching
Photobleaching is one of the banes of fluorescence microscopy. It's akin to watching your favorite movie and the screen suddenly dims – you want to see that vibrancy in your results! To combat this issue, a few tactics come in handy.
First off, using anti-fade reagents can provide a protective barrier for your fluorescent dye. Secondly, always limit the exposure to light before capturing your images; even a little care can go a long way in protecting your sample. Finally, selecting the right imaging settings can influence photobleaching. Utilizing lower laser power while extending exposure time is a valid strategy for minimizing such risks. In this arena, patience truly is a virtue.
Through these careful considerations – be it concentration, fixative choice, or minimizing photobleaching – effective use of phalloidin TRITC can lead to impressive insights into the cellular architecture and dynamism.
Limitations and Challenges
In the realm of cellular biology, utilizing phalloidin TRITC for actin staining is not without its own set of hurdles. Understanding these limitations grants researchers a clearer overview of how to interpret their data accurately, which is pivotal for deriving meaningful conclusions from experiments. Here, we dive into several specific areas of concern that can affect the efficacy and reliability of phalloidin TRITC staining.
Background Fluorescence Issues
One major complication in imaging with phalloidin TRITC is the phenomenon of background fluorescence. Background fluorescence occurs when non-specific signals interfere with the intended fluorescence. This can stem from sample preparation, the use of certain fixatives, or even the inherent properties of the biological materials under scrutiny. When background signals are present, it can obscure the true fluorescence emitted from the actin filaments, making analysis tricky.
To mitigate this challenge, researchers are encouraged to:
- Use appropriate controls: Incorporating negative controls during staining can help identify unwanted fluorescence.
- Optimize washing procedures: Ensuring thorough washing after staining can remove excess phalloidin, reducing background signal.
- Select suitable imaging settings: Adjusting laser intensity and filter sets according to the sample can help in distinguishing real signals from noise.
Variability in Actin Polymerization
Actin polymerization is a dynamic process that is influenced by numerous factors. This variability can introduce inconsistencies in staining results. Factors such as ion concentration, pH levels, and the presence of actin-binding proteins can affect how actin filaments assemble and disassemble. This means that the same sample might yield different staining results under varying experimental conditions.
Key considerations include:
- Environmental factors: Temperature and buffer composition must be kept consistent.
- Experimental protocols: Adhering strictly to the suggested incubation times and conditions while using phalloidin TRITC is crucial.
- Batch-to-batch variations: Variability can occur in actin polymerization across different preparations of the protein, leading to inconsistent results.
Interpretative Challenges in Results
Interpreting results from phalloidin TRITC staining can also present its set of challenges. The complexity of biological systems means that many factors can influence the observed fluorescence. For example, the density of actin networks can change depending on cellular context, possibly leading to misinterpretation of cellular dynamics or structure.
Thus, researchers must be well-versed in considering both qualitative and quantitative aspects. Use of software to analyze images quantitatively can aid in reducing subjective biases. However, it also raises the issue of potential software limitations, as not all software is equipped to accurately assess fluorescence under the wide variety of biological conditions encountered in practice.
To navigate these interpretive challenges:
- Cross-validation: Utilize complementary techniques (like live-cell imaging or Western blotting) to support findings.
- Community engagement: Sharing findings with peers or through forums may reveal overlooked inconsistencies or angles (see Reddit for discussions).
In summary, while phalloidin TRITC offers powerful tools for researchers studying actin dynamics, awareness of its limitations is vital. Understanding background fluorescence, the variability in actin polymerization, and the complexity of result interpretation can significantly enhance the reliability of conclusions drawn from experiments.
Thoughtful experimental design and diligent analysis remain key drivers in advancing this line of research.
Future Directions
The study of phalloidin TRITC is just starting to unfold its layers, revealing not just the mechanics of actin staining but also the evolving insights into cellular dynamics. The future of this tool intertwines with advancements in imaging technologies and novel applications in diverse research fields. Understanding these future directions will not only expand our grasp of cellular processes but also refine the techniques we employ in the lab.
Emerging Trends in Imaging Technologies
The realm of imaging technologies is witnessing a significant evolution, with several trends taking shape that could redefine how we understand and visualize cellular architectures. Two key directions include the rise of super-resolution microscopy and the integration of artificial intelligence (AI) in image analysis.
Super-resolution microscopy allows scientists to visualize actin filaments with unprecedented clarity, pushing the boundaries of the diffraction limit inherent in traditional microscopy. This technology employs various methods, from STED (Stimulated Emission Depletion) to SIM (Structured Illumination Microscopy), which can yield images with resolutions below 200 nanometers. As these methodologies develop, combining them with phalloidin TRITC staining could lead to groundbreaking discoveries in cell biology.


Additionally, AI's role in image processing is gaining traction, offering automated analysis that can overcome manual counting or qualitative assessments. This not only saves time but enhances the reproducibility of results. Algorithms can learn from vast datasets of stained samples, recognizing patterns in actin organization and facilitating a deeper understanding of cellular functions.
Potential for Novel Applications
The future beckons with numerous novel applications for phalloidin TRITC beyond mere visualization. As researchers continue to probe the molecular underpinnings of diseases, phalloidin TRITC can contribute significantly in areas like cancer research and neurobiology.
In cancer research, for example, understanding how actin filaments contribute to tumor cell invasion and metastasis becomes feasible. By employing phalloidin TRITC in live cell imaging, scientists can decipher the role of actin dynamics in cancer progression. This can lead to the formulation of targeted therapies aimed at disrupting these processes, potentially offering new avenues for treatment.
In neurobiology, the organization of actin in synaptic structures is critical to understanding neuronal communication. Phalloidin TRITC could facilitate live-cell studies, providing insights into how actin rearrangement affects synaptic plasticity. Gleaning knowledge at this level holds the promise for breakthroughs in neurodegenerative diseases where synaptic function is compromised.
Interdisciplinary Approaches in Research
The complex nature of cellular dynamics necessitates an interdisciplinary approach. Engaging experts from various fields—biologists, chemists, physicists, and data scientists—can foster innovation and expand the applications of phalloidin TRITC.
For instance, collaboration between chemical biologists and biophysicists can enhance how we synthesize and modify fluorescent tags like TRITC, optimizing their properties for specific applications. This collaboration leads to improved protocols in staining and visualization, catering to the unique requirements of different research arenas.
Furthermore, involving data scientists can significantly elevate our approaches to quantitative analysis in cellular imaging. Employing machine learning to correlate phalloidin TRITC staining with specific cell behaviors, one may derive meaningful insights that go beyond qualitative assessments.
By fostering an environment of shared knowledge, researchers can push the boundaries of our understanding of actin filaments in cellular contexts, laying the groundwork for future scientific breakthroughs.
In essence, the journey forward in regard to phalloidin TRITC is both exciting and broad, creating new pathways for exploration in the cellular compounds.
Epilogue
The conclusion serves as a critical piece of any discussion on Phalloidin TRITC, tying together the various threads explored throughout the article. It underscores the importance of understanding how this powerful tool reveals the intricate behaviors of actin filaments within cells. This section provides readers an opportunity to reflect on key findings about actin dynamics, the mechanisms by which phalloidin TRITC operates, and the practical applications that stem from these insights.
Synopsis of Key Insights
First and foremost, phalloidin TRITC beams a spotlight on the actin cytoskeleton, offering a window into cellular architecture. The insights gained through the use of this fluorescent labeling technique can be summarized as follows:
- Mechanistic Understanding: The article highlights how phalloidin binds specifically to F-actin, emphasizing the significance of this relationship in cellular structures.
- Polymerization Dynamics: The discussion on actin polymerization dynamics provides a framework for understanding how cellular morphology adapts during various processes such as motility and division.
- Advanced Imaging Capabilities: Our examination of cutting-edge imaging technologies illustrates how confocal and fluorescence microscopy can significantly enhance the visibility of actin structures, thereby enriching the quality of scientific research.
"The role of phalloidin TRITC transcends mere visualization; it acts as a vital tool for unraveling the complex dance of cellular events, paving the way for future discoveries."
Reflections on Phalloidin TRITC's Impact
The impact of phalloidin TRITC on cellular biology cannot be overstated. As researchers continue to dive deeper into the realm of cellular dynamics, the following aspects stand out:
- Enhancing Research Quality: The clarity and specificity offered by phalloidin TRITC vastly improve imaging quality, allowing for better delineation of cellular processes which are often obscured in traditional methodologies.
- Interdisciplinary Applications: The utilization of phalloidin TRITC extends beyond basic cellular studies, demonstrating its relevance in fields such as cancer research, neurobiology, and developmental biology.
- Innovative Approaches: With the advent of novel imaging techniques, future applications of phalloidin TRITC are bound to expand. These innovations promise to deepen our understanding of the actin cytoskeleton and its influence on various cellular functions.
In summary, the exploration of phalloidin TRITC provides important insights that advance the understanding of actin’s role in cellular dynamics. Its ability to assist in visualizing actin filaments offers valuable contributions to numerous research fields, solidifying phalloidin TRITC's position as an indispensable asset in modern cellular biology.
References and Further Reading
When navigating the complex realm of cell biology research, particularly concerning phalloidin TRITC and its role in actin staining, having robust references and further reading is essential. These sources not only fortify the foundation of knowledge but also illuminate the various dimensions of this subject. Let’s take a closer look at the elements that make these resources invaluable for students, professionals, and researchers alike.
Firstly, the importance of having well-documented studies and reviews cannot be overstated. They offer comprehensive analyses that can span from foundational knowledge to recent advancements in imaging techniques. By perusing these materials, readers gain insights that are deeper and more nuanced than a surface-level glance could ever provide. A thorough understanding of primary literature is key in building sound research methodologies and interpreting results accurately.
Moreover, the benefits of accessing a diverse range of references include:
- Enhanced comprehension of the mechanisms underlying actin dynamics.
- Understanding contextual applications of phalloidin TRITC beyond the laboratory, linking them to real-world issues in cellular biology.
- Comparison of different techniques and methodologies employed in active research.
In addition to the core studies directly related to phalloidin, exploring literature that discusses imaging technologies and methodologies provides crucial context. As the field evolves, being aware of cutting-edge techniques allows researchers to leverage novel approaches in their own work.
An important consideration with references is assessing their reliability and accuracy. Peer-reviewed articles typically offer the most authoritative information. Moreover, secondary sources like textbooks can provide foundational knowledge but should be supplemented with contemporary research for a broader perspective. Established publishers, such as Elsevier or Wiley, are good starting points for reliable research.
Key Resources
It’s beneficial to stay tied to reputable databases and journals as you explore further. Here are some key platforms that could be of interest:
- PubMed: A robust database for life sciences that provides access to a vast number of peer-reviewed articles.
- Google Scholar: This can be a treasure trove for finding a wide range of studies, some older ones could provide context missing in contemporary writing.
- ResearchGate: An excellent platform for connecting with authors who have published work on phalloidin TRITC, allowing for direct inquiries and collaborations.
In sum, references and further reading on phalloidin TRITC encapsulate the essence of informed research. Not only do they provide a foundation of knowledge, but they also encourage critical thinking and a deeper engagement with material that shapes our understanding of cellular dynamics. The more familiar one becomes with existing literature, the stronger and more innovative research practices can be developed.
Key Studies on Phalloidin TRITC
A delve into key studies reveals the breadth of application and research surrounding phalloidin TRITC. Such studies articulate the practicality and significance of this compound in the visualization of actin filaments. Noteworthy literature includes:
- The Initial Discoveries: Early studies focusing on the binding properties of phalloidin to F-actin elucidated the compound's pivotal role in successfully visualizing the actin cytoskeleton.
- Comparative Analyses: Certain analyses compare different staining agents in regards to specificity and signal strength, positioning phalloidin TRITC as a frontrunner in providing clarity and detail.
- Development of Techniques: Several studies pioneer techniques in imaging that harness the power of phalloidin TRITC, illustrating how advancements in microscopy methods can enhance imaging quality and reproducibility.
These studies act as a cornerstone for researchers aiming to employ phalloidin TRITC effectively. They provide not just methodology, but also discussion on the implications of findings which may influence future directions in research.
Resources for Advanced Research
In the pursuit of advanced research, leveraging a plethora of resources is critical. Scholars and practitioners can benefit from an array of tools and collections that provide both foundational theory and new methodologies. Recommendations for deepening your research include:
- Online Database Access: Leveraging university library resources can link researchers to subscriptions for journals rich in articles about phalloidin TRITC.
- Collaboration Platforms: Networks such as LinkedIn can connect researchers globally, fostering dialogues that may lead to collaborative research endeavors.
- Conferences and Symposia: Participating in relevant conferences can expose you to the latest studies and enable discussions with field experts which can serve to inspire your own research initiatives.
Utilizing these resources will certainly create a well-rounded approach to the complexities surrounding phalloidin TRITC, ensuring that you are both informed and up-to-date on current trends and breakthroughs. By integrating these insights into your research practice, you can cultivate a more profound understanding of actin dynamics and the essential role of imaging techniques in cell biology.







